Resize Your Font
Patients & Caregivers
Resize Your Font
February is CCA Awareness Month. Event Page
Cholangiocarcinoma (CCA)
(Bile Duct Cancer)
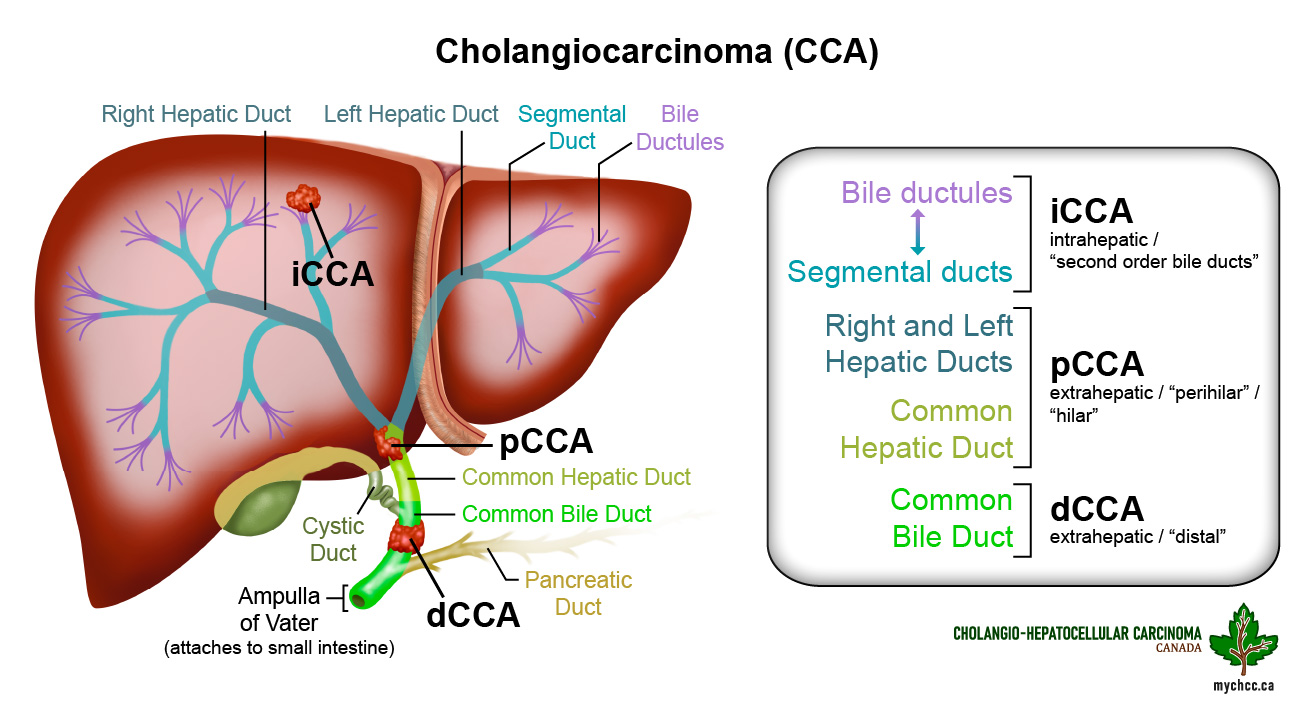
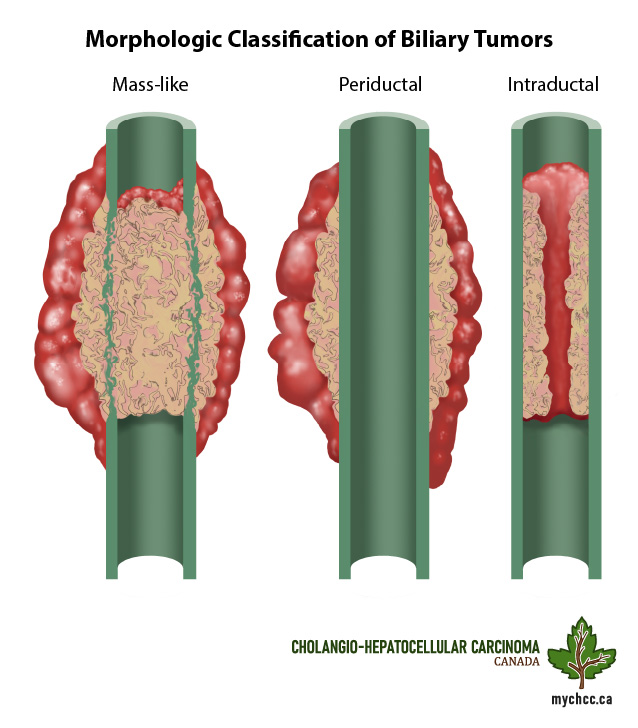
What is cholangiocarcinoma?
- It is a cancer of the bile ducts.
- There are three different types of cholangiocarcinoma (CCA), based on location:
- Intrahepatic cholangiocarcinoma (iCCA) found in bile ducts within the liver.
- Extrahepatic or distal cholangiocarcinoma (dCCA) found in the part of the bile ducts outside the liver.
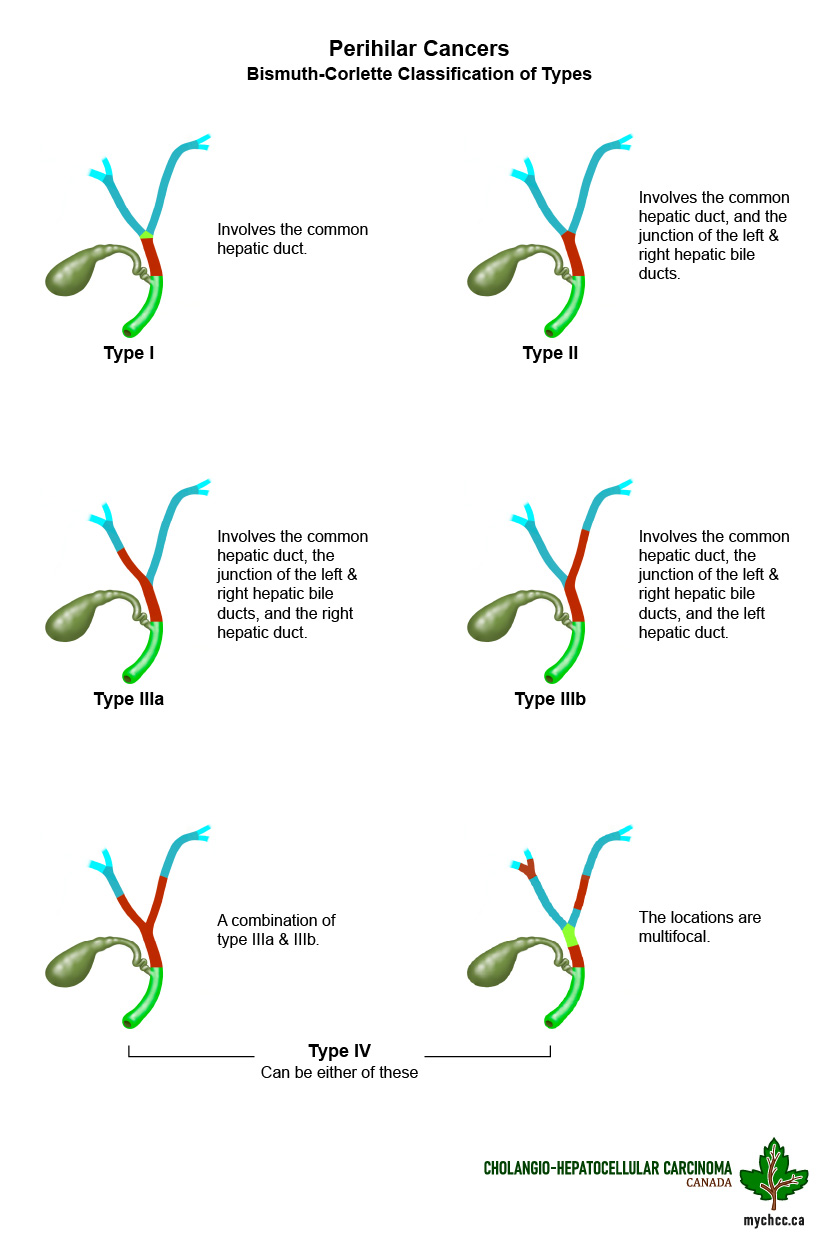
- Perihilar cholangiocarcinoma (pCCA) or Klatskin’s tumor where the right and left bile ducts join.
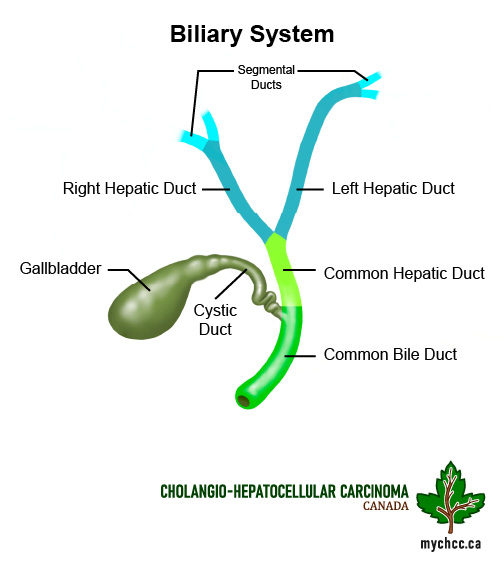
What do the bile ducts do?
- The bile ducts are the tubes that connect the liver and gall bladder to the small intestine.
- Bile is produced in the liver and the bile ducts allow bile to be delivered from the liver and gall bladder to the small bowel to aid in fat digestion.
Illustrations
Illustration by David R. Purnell, MA, AMI
Illustration by David R. Purnell, MA, AMI
Illustration by David R. Purnell, MA, AMI
Illustration by David R. Purnell, MA, AMI
Risk Factors
Most CCA occur without a distinguishing cause.
- Primary sclerosing cholangitis (PSC), an autoimmune disorder
- Primary biliary cholangitis (PBC)
- Chronic viral infection with Hepatitis B or C
- Ulcerative colitis
- Liver flukes (parasitic worms)
- Diabetes
- Chemical exposure
- Congenital conditions: Abnormal joining of the bile and pancreatic ducts and Choledochal cysts, abnormal dilations of the bile ducts outside the liver (rare)
- Obesity
- Alcohol consumption
- Tobacco smoking
- Cirrhosis of the liver
- Non-alcoholic fatty liver disease (NAFLD), now called metabolic dysfunction-associated steatotic liver disease (MASLD)
- Metabolic syndrome
Signs and Symptoms
- Jaundice (yellowing of the skin and eyes)
- Dark urine
- Clay coloured stool
- Pain in the abdomen
- Itchy skin
- Fever
- Nausea and vomiting
- Fatigue
- Weight loss
Diagnosis
- Blood chemistry tests
- Tumor marker tests. CEA and CA-19-9
- Biopsy
- Ultrasound
- CT scan
- Laparoscopy
- Biomarkers
Biomarkers
- Liquid biopsy. Serum and urine tests that could show circulating DNA (ctDNA) biomarkers present in the tumor tissue, making it a plausible method to diagnose CCA earlier.
- Genomic profiling from a validated lab. ‘Oncohelix’ in Calgary is a licensed lab who can perform these tests. The biomarkers most common for CCA include: BRAF, FGFR2, ERBB2, IDH1, IDH2, MLD1, MSH6, NTRK1, NTRK2, NKRK3, PALB2, PMS2, RET, BRAC1, BRAC2 (Oncohelix, 2023). If one of these is not present, a more comprehensive genomic profiling will have to be done.
Treatment
- Surgery - removal of part of the bile duct if the tumor is small or partial hepatectomy or Whipple procedure.
- Adjuvant therapy after surgery; chemotherapy or radiation therapy to lower risk of cancer returning
- Palliative surgery - Biliary bypass or Endoscopic stent placement
- Radiation therapy
- Chemotherapy used drugs to stop the growth of cancer cells
- Standard first line treatment in Canada is gemcitabine-cisplatin plus immunotherapy (durvalumab) (Ramjeesingh et al., 2023)
- Regional chemotherapy
- Immunotherapy
- CAR-T therapy
- Targeted Therapy
- Histotripsy
- Y-90 radioembolization
Prevention
- Stop smoking
- Reduce risk of liver disease: drink alcohol in moderation (0-2 drinks /week)
- Maintain a healthy weight
- Cook meat thoroughly
- Follow directions when working with chemicals
Prognosis
- Aggressive cancer with a median survival rate of 6-12 months, if unresectable at diagnosis.
- The only cure at this time is totally resectable tumor.
- Globally the 5 year survival rate is 10%.
Printable Booklet (PDF)
- Printable CCA booklets are available in the following languages:
- English
- French (Français)
- Farsi فارسی
- Hindi हिंदी
- Italian (Italiano)
- Punjabi ਪੰਜਾਬੀ
- Spanish (Español)
- Tagalog
- Ukrainian українська
- Simplified Chinese 简体中文
- Traditional Chinese (Hong Kong) 傳統中文 (香港)
- Traditional Chinese (Taiwan) 傳統中文 (臺灣)
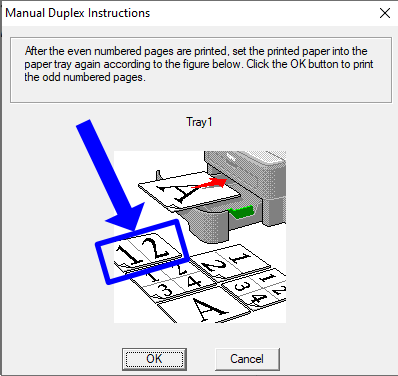
- When printing, choose "Print on both sides of paper" and "Flip on short edge."
- When flipping the paper, be sure to follow the correct orientation for your printer. An example is shown below, circled in blue.
- Note: The image is just an example. The orientation may be different for your printer. When the page flip dialogue appears, follow the "1|2" landscape orientation given by your printer, not this image.
References
Canadian Cancer Society (2023). What is bile duct cancer? Retrieved from:
https://cancer.ca/en/cancer-information/cancer-types/bile-duct
Cancer.Net Editorial Board (2021). Bile duct cancer (Cholangiocarcinoma). Cancer.Net: American Society of Clinical Oncology (ASCO). Retrieved from: https://www.cancer.net/cancer-types/bile-duct-cancer-cholangiocarcinoma
Gupta, A. & Dixon, E. (2017). Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surgery and Nutrition, 6(2): 101-104 doi: 10.21037/hbsn.2017.01.02
Kirstein , M.M. & Vogel, A. (2016) Epidemiology and Risk factors of cholangiocarcinoma. Visceral Medicine 32(6). 395-400. doi: 10.1159/000453013
Oncohelix (2023). Retrieved from https://oncohelix.org/2023/03/01/press-release-avenio-324-gene-cgp-panel-matched-to-foundationone-cdx-is-now-available-in-canada/
Mayo Clinic (2023). Cholangiocarcinoma (bile duct cancer). Retrieved from: https://www.mayoclinic.org/diseases-conditions/cholangiocarcinoma/symptoms-causes/syc-20352408
National Cancer Institute (2022). What is bile duct cancer (Cholangiocarcinoma)? Retrieved from: https://www.cancer.gov/types/liver/bile-duct-cancer
Ramjeesingh, R., Chaudhury, P., Tam, V.C., Roberge, D., Lim, H.J., Knox, J.J., Asselah, J., Doucette, S., Chhiber, N., Goodwin, R. (2023). A practical guide for the systemic treatment of biliary tract cancer in Canada. Current Oncology. 30, 7132-7150. doi.10.3390/curroncol30080517. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/37622998/
Cancer.Net Editorial Board (2021). Bile duct cancer (Cholangiocarcinoma). Cancer.Net: American Society of Clinical Oncology (ASCO). Retrieved from: https://www.cancer.net
Gupta, A. & Dixon, E. (2017). Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surgery and Nutrition, 6(2): 101-104 doi: 10.21037/hbsn.2017.01.02
Kirstein , M.M. & Vogel, A. (2016) Epidemiology and Risk factors of cholangiocarcinoma. Visceral Medicine 32(6). 395-400. doi: 10.1159/000453013
Oncohelix (2023). Retrieved from https://oncohelix.org
Mayo Clinic (2023). Cholangiocarcinoma (bile duct cancer). Retrieved from: https://www.mayoclinic.org
National Cancer Institute (2022). What is bile duct cancer (Cholangiocarcinoma)? Retrieved from: https://www.cancer.gov
Ramjeesingh, R., Chaudhury, P., Tam, V.C., Roberge, D., Lim, H.J., Knox, J.J., Asselah, J., Doucette, S., Chhiber, N., Goodwin, R. (2023). A practical guide for the systemic treatment of biliary tract cancer in Canada. Current Oncology. 30, 7132-7150. doi.10.3390/curroncol30080517. Retrieved from: https://pubmed.ncbi.nlm.nih.gov
Disclaimer: The information on this website is for educational purposes only. Please consult your doctor for professional medical advice.
©2026 Cholangio-Hepatocellular Carcinoma Canada
Charitable Registration Number 709631014 RR 0001
©2026 Cholangio-Hepatocellular Carcinoma Canada
Charitable Registration Number 709631014 RR 0001